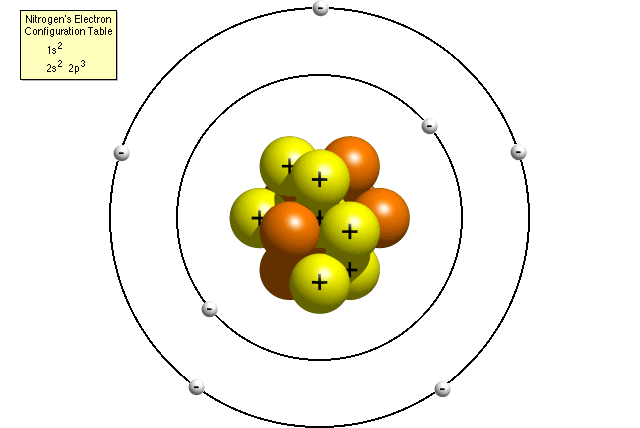
A Bohr model of a nitrogen atom

Although superseded by the Quantum model, Bohr's model
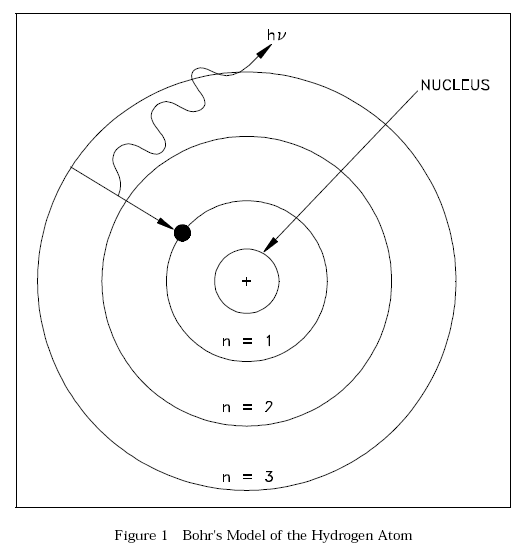
Bohr Model of the Atom

In 1913, Niels Bohr devised Bohr's atomic model

Bohr's atom model

building an atom model. He also showed that electrons can move from a lower
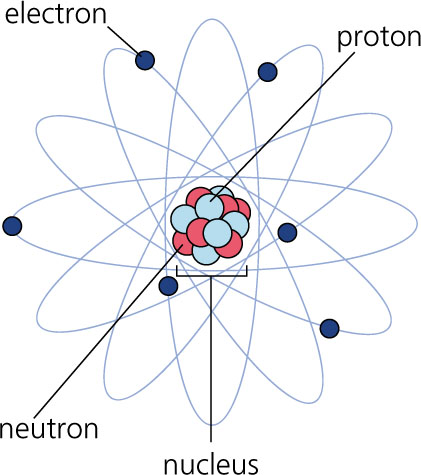
Quantum physics has something else to say about the atomic model.

Bohr's atomic model describes the structure of an atom as an atomic nucleus
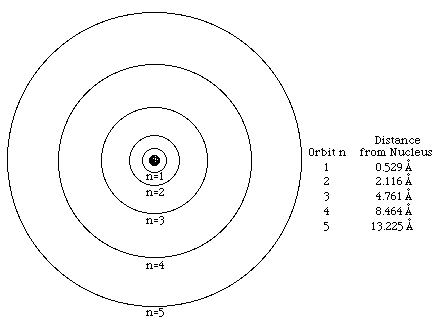
The Bohr model of the atom.

With his model, Bohr explained how electrons could jump from one orbit to

Bohr atomic model:

Neils Bohr - Atomic Model

Bohr models

the classic atomic models (Dalton, Thomson, Rutherford and Bohr) and

A typical model of the atom is called the Bohr Model,

Bohr atom model in which h is Planck's constant, m is the mass of the

The Bohr Model of the Atom. 1913, Bohr speculated that electrons orbit

The Bohr model of the atom is not perfect, but we don't casually dismiss it
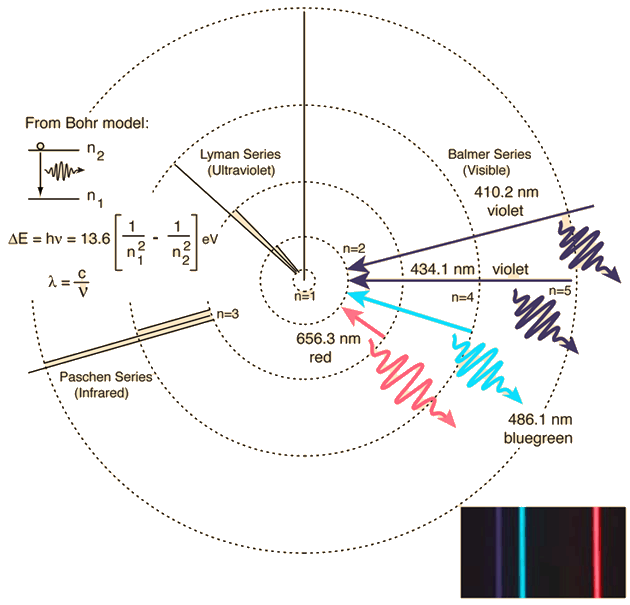
This spectrum was produced by exciting a glass tube

In the original Bohr atomic model there was not an explanation
No comments:
Post a Comment